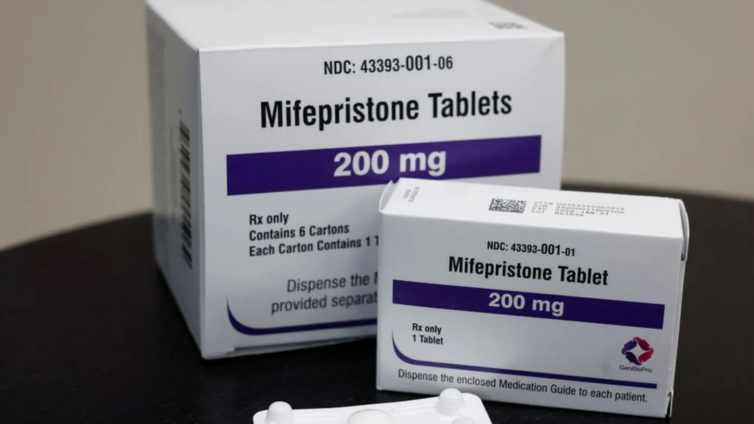
The US Supreme Court will hear oral arguments on whether to restrict access to mifepristone, a commonly used abortion pill.
It is considered the most significant reproductive rights case since the court ended the nationwide right to abortion in June 2022.
The Biden administration hopes the court will overturn a decision to limit access to the drug over safety concerns raised by anti-abortion groups.
The pill has been legal since 2000.
The current legal battle in the top US court began in November 2022 when the Alliance for Hippocratic Medicine, an umbrella group of anti-abortion doctors and activists, filed a lawsuit against the Food and Drug Administration, or FDA.
The group claims that mifepristone is unsafe and further alleges that the federal agency unlawfully approved its use in September 2000 to medically terminate pregnancies through seven weeks gestation.
Mifepristone is used in combination with another drug - misoprostol - for medical abortions, and it is now the most common way to have an abortion in the US.
Medical abortions accounted for 63% of all abortions in 2023, up from 53% in 2020, according to the Guttmacher Institute.
In total, more than five million US women have used mifepristone to terminate their pregnancies.
The court has previously ruled that it would not consider a challenge of the FDA's approval of the drug. This case will instead centre on the federal agency's decision to loosen restrictions of its use since 2016.
The FDA announced in 2016 that it would allow mifepristone's use until the 10-week mark, and then in 2021 it lifted in-person dispensing requirements - a move that allowed providers to send it to patients by mail.
In 2022, the FDA moved further by allowing retail pharmacies to dispense the drug, meaning medical professionals - not just doctors - could prescribe it. The following year, a judge in Texas revoked the FDA's approval of mifepristone.
Now, the nine judges of the conservative-leaning Supreme Court must first determine whether the Alliance for Hippocratic Medicine can legally challenge the FDA in court, before ultimately deciding on whether the FDA's changes to access were lawful.
Its decision could force the FDA to tighten the restrictions again and make it harder for Americans to access the pill.
Marsha Henderson, the former FDA associate commissioner for women's health, said that reversing the 2016 and 2021 changes "will make reproductive care more resource-intensive and less safe".
Ms Henderson noted that mifepristone is regulated "more strictly and studied more intensely" than most other drugs. She added that a reversal would "undermine the FDA's congressionally granted authority and confuse patients".
Latest Stories
-
Muntaka vows crackdown on prostitutes, beggars in five cities
6 minutes -
Arteta keen to keep Partey at Arsenal amid transfer interest
26 minutes -
Accra Floods: Not just rain, but negligence
37 minutes -
Adjaye Associates designs pioneering Cancer Research Hub for children in Ghana
45 minutes -
Arteta wants ‘important player’ Thomas Partey to stay at Arsenal
49 minutes -
We Move the Game: Malta Guinness celebrates a powerful season finale of Women’s Premier League
1 hour -
Godfred Yeboah Dame reported to General Legal Council for alleged misconduct
1 hour -
Prof Francis Otoo appointed Ag. Director-General of Nuclear Regulatory Authority
1 hour -
Tulenkey explains why he taped his mouth at 26th TGMAs
1 hour -
KATH launches orthopaedics month to fund free surgeries and raise bone health awareness
1 hour -
Inclusive business models key to Ghana’s agribusiness growth – Chamber of Young Entrepreneurs
1 hour -
Finance Ministry approves revised allowances for university senior staff unions
2 hours -
Nhyira FM’s Power Sports Host goes ‘Sakora’ on live radio after Manchester United defeat
2 hours -
ECG to begin maintenance works for sustained power supply in Ashanti as the rains set in
2 hours -
My mum told me to quit music – Wendy Shay
2 hours