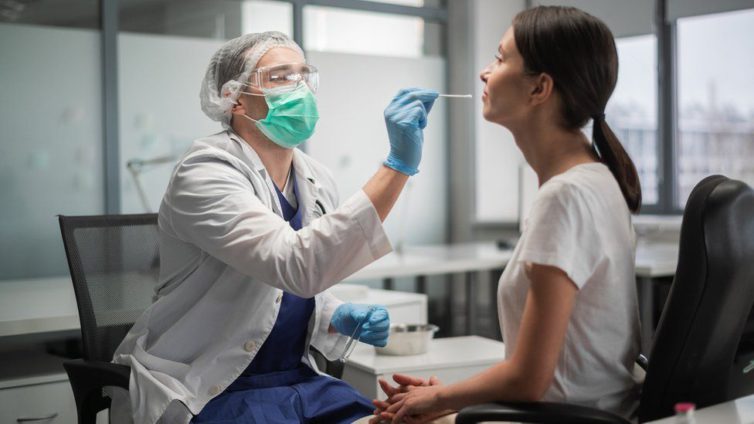
This monoclonal antibody – the second to be authorised by the Medicines and Healthcare products Regulatory Agency – is for people with mild to moderate COVID-19 who are at high risk of developing severe disease.
Another COVID-19 treatment, Xevudy (sotrovimab), has today been approved by the Medicines and Healthcare products Regulatory Agency (MHRA) after it was found to be safe and effective at reducing the risk of hospitalisation and death in people with mild to moderate COVID-19 infection who are at an increased risk of developing severe disease.
This follows a rigorous review of its safety, quality and effectiveness by the UK regulator and the government’s independent expert scientific advisory body, the Commission on Human Medicines, making it the second monoclonal antibody therapeutic to be approved following Ronapreve.
Developed by GSK and Vir Biotechnology, sotrovimab is a single monoclonal antibody. The drug works by binding to the spike protein on the outside of the COVID-19 virus. This in turn prevents the virus from attaching to and entering human cells, so that it cannot replicate in the body.
In a clinical trial, a single dose of the monoclonal antibody was found to reduce the risk of hospitalisation and death by 79% in high-risk adults with symptomatic COVID-19 infection.
Based on the clinical trial data, sotrovimab is most effective when taken during the early stages of infection and so the MHRA recommends its use as soon as possible and within five days of symptom onset.
Like molnupiravir, it has been authorised for use in people who have mild to moderate COVID-19 infection and at least one risk factor for developing severe illness. Such risk factors include obesity, older age (>55 years), diabetes mellitus, or heart disease.
Unlike molnupiravir, sotrovimab is administered by intravenous infusion over 30 minutes. It is approved for individuals aged 12 and above who weigh more than 40kg.
It is too early to know whether the omicron variant has any impact on sotrovimab’s effectiveness but the MHRA will work with the company to establish this.
Dr June Raine, MHRA Chief Executive said:
“I am pleased to say that we now have another safe and effective COVID-19 treatment, Xevudy (sotrovimab), for those at risk of developing severe illness.
“This is yet another therapeutic that has been shown to be effective at protecting those most vulnerable to COVID-19, and signals another significant step forward in our fight against this devastating disease.
“With no compromises on quality, safety and effectiveness, the public can trust that the MHRA have conducted a robust and thorough assessment of all the available data.”
Professor Sir Munir Pirmohamed, Chair of the Commission on Human Medicines, said:
“The Commission on Human Medicines and its COVID-19 Therapeutics Expert Working Group has independently reviewed the data and agrees with the MHRA’s regulatory approval of Xevudy (sotrovimab).
“When administered in the early stages of infection, sotrovimab was found to be effective at reducing the risk of hospitalisation and death in high-risk individuals with symptomatic COVID-19. Based on the data reviewed by the Commission and its expert group, it is clear sotrovimab is another safe and effective treatment to help us in our fight against COVID-19.”
Sotrovimab is not intended to be used as a substitute for vaccination against COVID-19.
The government and the NHS will confirm how this COVID-19 treatment will be deployed to patients in due course.
Latest Stories
-
Bayer Leverkusen’s Jeremie Frimpong arrives in Ghana for visit
4 minutes -
‘It will be disastrous if Mahama removes the Chief Justice’ – Prof. Stephen Adei
6 minutes -
Jean Mensa must step down as EC Chair – APC and Movement for Change assert
25 minutes -
Akufo-Addo calls on police to refine strategies to avoid prolonged electoral unrest
29 minutes -
Only NPP looting brigade unhappy about ORAL – Ablakwa
31 minutes -
CSIR-SARI introduces integrated soil fertility management technology to boost maize production
32 minutes -
Ghana’s indigenous agribusiness faces challenges impacting economic growth – Dr. Azinu
34 minutes -
41-year-old man arrested over illegal power connection
36 minutes -
65-year-old man plans to walk over 250-km Kumasi-Accra journey for Mahama’s swearing-in
37 minutes -
Woman dies after being set on fire on NYC subway
2 hours -
Elon Musk’s curious fixation with Britain
2 hours -
EBID wins the Africa Sustainability Award
4 hours -
Expansion Drive: Takoradi Technical University increases faculties
8 hours -
SHS heads demand payment of outstanding funds before reopening of schools
9 hours -
We thank God for the 2024 general elections – Akufo-Addo
9 hours