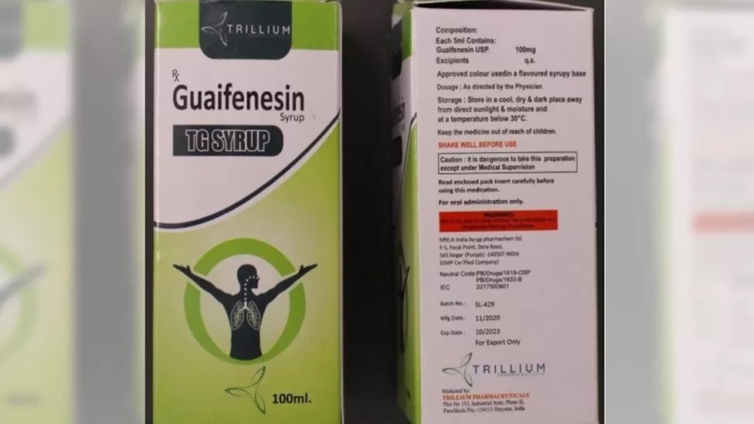
The World Health Organization has said that a batch of contaminated India-made cough syrup has been found in the Marshall Islands and Micronesia.
The WHO said that the tested samples of Guaifenesin TG syrup, made by Punjab-based QP Pharmachem Ltd, showed "unacceptable amounts of diethylene glycol and ethylene glycol".
Both compounds are toxic to humans and could be fatal if consumed.
The WHO statement did not specify if anyone had fallen ill.
The latest alert comes months after the WHO linked other cough syrups made in India to child deaths in The Gambia and Uzbekistan.
Sudhir Pathak, managing director of QP Pharmachem, told the BBC that the company had exported the batch of 18,346 bottles to Cambodia after getting all due regulatory permissions. He said he didn't know how the product had reached the Marshall Islands and Micronesia.
"We did not send these bottles to the Pacific region, and they were not certified for use there. We don't know under what circumstances and conditions these bottles reached the Marshall Islands and Micronesia," he said, adding that his company has sent a legal notice to the firm that exported the batch of medicines to Cambodia.
The WHO statement said that the product, which is used to relieve chest congestion and cough symptoms, was tested by Australia's drug regulator, the Therapeutic Goods Administration.
The syrup was marketed by Trillium Pharma, based in Haryana state. The BBC couldn't reach a Trillium representative on the phone. The Indian government has not reacted to the latest alert.
The statement added that "neither the stated manufacturer nor the marketer have provided guarantees to WHO on the safety and quality of these products".
India is the world's largest exporter of generic drugs, meeting much of the medical needs of developing countries.
But in recent months, many Indian firms have come under scrutiny for the quality of their drugs, with experts raising concerns about the manufacturing practices used to make these medicines.
In October, WHO had sounded a global alert and linked four cough syrups made by Maiden Pharmaceuticals to the deaths of 66 children from kidney injuries in The Gambia.
Both the Indian government and the company, Maiden Pharmaceuticals, had denied the allegations.
In March, India cancelled the manufacturing licence of a firm whose cough syrups were linked to 18 child deaths in Uzbekistan. Earlier this month, the FDA said it had found that the Indian manufacturer of eye drops linked to three deaths and serious infections in the US had violated several safety norms.
Latest Stories
-
Drama as police corner armed robbers inside locked forex bureau at Lapaz
4 mins -
Nigerian-born conquers childhood hearing loss to become KNUST’s overall best graduating student
30 mins -
ECOWAS Court orders compensation for violations against New Force’s Shalimar Abbiusi
42 mins -
Dreams FC denies allegations of attempting to sign Najeeb Yakubu
2 hours -
Election 2024: ‘Right to free and fair elections non-negotiable’ – Akufo-Addo
2 hours -
Kurt Okraku took out my passport from the U23 squad that travelled to Japan – Najeeb Yakubu alleges
2 hours -
Where hope fails: Ghana’s decaying home for the destitute
2 hours -
NDC Mining Committee for 2024 campaign refutes allegations of recruiting thugs for elections
2 hours -
Traction Control: A lifesaver with an off switch? Here’s why it exists
2 hours -
I don’t need anyman to woo me with money – Miss Malaika 2024 winner refutes pimping claims
2 hours -
”Kurt Okraku sabotaged my national team career because I refused to sign with Dreams FC” – Najeeb Yakubu
2 hours -
Businesses urged to leverage Generative AI for enhanced customer engagement
3 hours -
MultiChoice Ghana partners with Ghana Hotels Association to elevate guest entertainment
3 hours -
Bawumia’s music streaming app or Mahama’s pay-per-view TV channel?
3 hours -
Karpowership Ghana empowers 40 Takoradi Technical University students with scholarship
3 hours