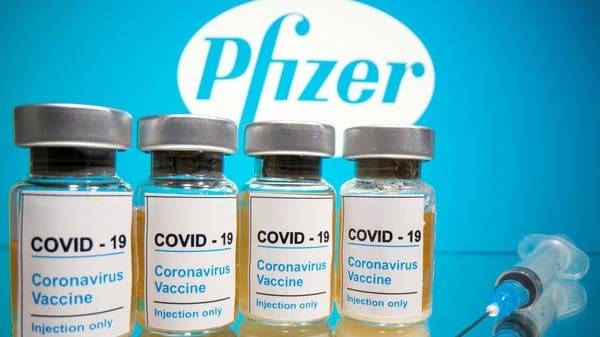
Pfizer expects to file for full US Food and Drug Administration approval of its Covid-19 vaccine for 16-to-85-year-olds by the end of May, the company said during its first quarter 2021 earning teleconference Tuesday.
“While we are currently distributing our vaccine in the US under an Emergency Use Authorization (EUA), we expect to submit this month a Biologics License Application (BLA) to the US Food and Drug Administration (FDA) seeking full approval for our Covid-19 vaccine for individuals 16 years of age and older,” Pfizer CEO Albert Bourla said in his remarks.
DISCLAIMER: The Views, Comments, Opinions, Contributions and Statements made by Readers and Contributors on this platform do not necessarily represent the views or policy of Multimedia Group Limited.
Latest Stories
-
DAMC, Free Food Company, to distribute 10,000 packs of food to street kids
1 hour -
Kwame Boafo Akuffo: Court ruling on re-collation flawed
1 hour -
Samuel Yaw Adusei: The strategist behind NDC’s electoral security in Ashanti region
1 hour -
I’m confident posterity will judge my performance well – Akufo-Addo
2 hours -
Syria’s minorities seek security as country charts new future
2 hours -
Prof. Nana Aba Appiah Amfo re-appointed as Vice-Chancellor of the University of Ghana
2 hours -
German police probe market attack security and warnings
2 hours -
Grief and anger in Magdeburg after Christmas market attack
2 hours -
Baltasar Coin becomes first Ghanaian meme coin to hit DEX Screener at $100K market cap
3 hours -
EC blames re-collation of disputed results on widespread lawlessness by party supporters
4 hours -
Top 20 Ghanaian songs released in 2024
4 hours -
Beating Messi’s Inter Miami to MLS Cup feels amazing – Joseph Paintsil
4 hours -
NDC administration will reverse all ‘last-minute’ gov’t employee promotions – Asiedu Nketiah
4 hours -
Kudus sights ‘authority and kingship’ for elephant stool celebration
4 hours -
We’ll embrace cutting-edge technologies to address emerging healthcare needs – Prof. Antwi-Kusi
5 hours