A curious thing happened about a week ago. The Government of Ghana, which has been touting its investments into Covid-19 testing capacity to universal acclaim, as the country galloped up the continental league tables, suddenly found itself in the dock.
Before long, prominent health leaders in the country were accusing the Government of massaging testing numbers for PR benefits.
Ghana’s magic
By the time the dust settled, it had become clear that it wasn’t massive investments into diagnostic assays and reagents, not to talk of RT-PCR testing machines, that had catapulted Ghana to its celebrated position of number two in the league table of African countries that have carried out the most tests. Rather, it was the ingenuity of scientists at the country’s preeminent biomedical institution, the Noguchi Memorial Institute of Medical Research that was largely responsible.
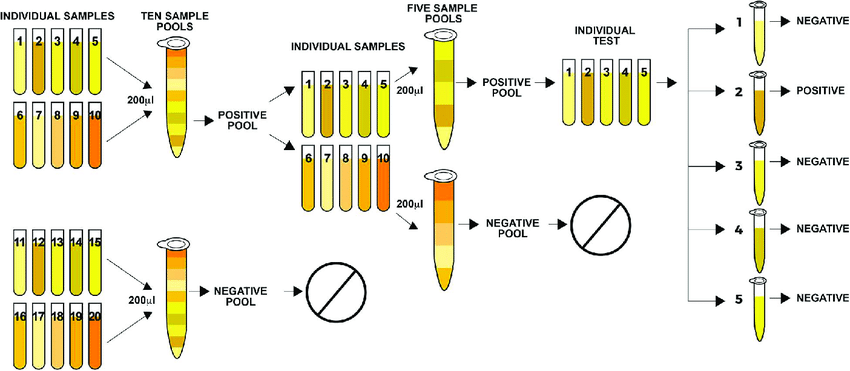
Without waiting for the usual bureaucracy of national ethical review approvals or situation-specific peer-reviewed studies, they had decided to deploy the well-known process of “pooled sampling”, thereby expanding testing capacity severalfold literally overnight. I chronicle these fascinating matters here and here.
With countries like Nigeria struggling to obtain enough reagents and other testing consumables in the international market, and with their testing output stuck at 0.0055% of the population (compared to Ghana’s 0.328%, which is nearly 60 times higher on a proportional basis), Ghana’s feats had seemed like pure magic.
A matter of some urgency
The Africa Centers for Disease Control (Africa CDC), the lead continental agency coordinating the regional Covid-19 response, in its 23rd April situation report, gave the total number of tests conducted on the continent as 415,000, of which Ghana alone was responsible for at least a sixth. By the 27th of April, Ghana had completed over 100,000 tests, whilst Nigeria was hovering around 12,000 tests. The Head of the Nigerian CDC continued to open up about the challenges the country was having in securing supplies of reagents because of severe global shortages.
Recognising that Ghana’s ten-fold multiple of Nigeria’s testing capacity is almost entirely down to a clever algorithm – testing based on pooled samples – one immediately begins to wonder why other African countries should not immediately adopt this algorithm and accelerate their testing coverage dramatically. Especially bearing in mind that with the worldwide testing average now in the region of 1.5%, Africa’s less than 0.01% is no longer tenable. Should Africa not embrace pooled testing continentally right away?
Perhaps, we first need to break down the principles of pooled sampling. When I touched on the subject in my earlier articles, many people reached out to me on social media and through my blog to challenge my thinking and ask clarification questions about my analysis. Clearly, it is the most critical short-term decision African public health authorities would need to make regarding how to alleviate the constraints holding back mass testing.
Pooled testing: The basics
Supposed you had 100 items you needed to test. You know that a couple of these items have a particular defect but you don’t know which specific items. You could certainly test each item one after the other. But knowing that only a few of these items have the defect, that would seem to be a waste of time and resources, especially if each test consumes significant time and resources.
Supposed the items could be mixed up in such a manner that the resulting mixture was generally similar in properties to the individual items? If so, then it would make sense to group the 100 items into smaller pools of ten items each (leaving a reserve amount from each sample) and proceed to test each pool. If any of the pools test positive for the defect in question, then the reserve samples from only that pool can be tested one after the other to find out which sample or samples from that pool might contain the defect that is triggering the positive result.
This algorithm can be summarised very simply, if also crudely, as follows.
| Number of Samples | 100 |
| Test Round | 1 |
| Number of Pools | 10 |
| Number of Tests | 10 |
| Pools Testing Positive | 2 |
| Tests “Wasted” | 8 |
| Test Round | 2 |
| Number of Pools | 0 |
| Number of Tests | 20 |
| Tests Returning Positive | 3 |
| Positive Case Rate | 3% |
| Tests “Wasted” | 17 |
| Total Number of Tests | 30 |
| Tests Saved | 70 |
In effect, assuming simplistically that one homogenous test kit is required for each testing exercise, 30 test kits have been used to do what would have required 100 test kits in the conservative one-by-one model.
More Math Than Biology
The first thing that may have jumped at the reader is the statistical nature of the decision-making here. It is not molecular virology that determines, fundamentally, the issues at play. True, that important discipline plays a role in addressing some of the concerns about sensitivity (a measure of how effective a testing method is in fishing out genuinely or truly defective samples) and specificity (a measure of how effective a testing method is in establishing that no defect exists) that the rest of this article will grapple with, but the primary logic derives from statistical analysis. Not surprising then that the first person to rigorously publish about the process was a political economist writing for a statistical journal.
Sensitivity
Only a tiny modicum of imagination is needed to spark the most obvious concern: might mixing the ten samples into one pool not dilute it to the point where it falls below the threshold of detection? In lay terms, and in the context of Covid-19, could it not happen that when a small quantity from one or more original samples is scooped out and mixed with others to create a new single sample (“the pool”), that smaller sub-quantity might lack the viral RNA? This is the not-so-difficult-to-fathom issue of dilution. Because the tube or well in which each test reaction takes place inside the PCR machine is finite in size, pooling samples necessarily means reducing the amount of sub-sample taken from each original sample. RT-PCR tests may be the gold standard for detection now, but they are certainly not perfect. There is a roughly 10% chance anytime a PCR test is conducted that the result may be invalid. Will dilution not compound matters?
Luckily, there has been quite a steady stream of experimental research work that has established that modern assays are now so sensitive that the dilution risk is not necessarily elevated by pooling samples, though considerable optimisation is required for different infection scenarios.
So, all good then, we can effectively expand capacity by up to ten times across Africa by instituting pooling as a continental protocol. Except that there are some very important caveats to consider before celebrating this potential bonanza.
Pooling Efficiency
As already indicated, the algorithmic logic of pooling is based on simple mathematics, but one that can become very complex very quickly. I will spare the reader the exotic combinatorics and focus on the easy matter of “pre-test probability”.
Because of weeks of conducting Covid-19 tests, every country now has a rough idea of what the probability of detecting a positive case might be at every point in time. While this factor is changing all the time, the shifts are fairly steady and programmable. In Nigeria, for instance, the probability that a test will return positive is nearly 9.5%. In Ghana, it is currently around 1.5%. The vast disparity is significant and will be touched on later.
This “likelihood of finding a defect” (or, in the Covid-19 context, an individual testing positive) has a huge effect on the effectiveness of pooled sampling. Let us return to our simple table above. The positive rate in that thought experiment was 3%. But supposed we brought it closer to the Nigerian case, and assumed a 9% pre-test probability of finding a defect (or positive case)?
| Number of Samples | 100 |
| Test Round | 1 |
| Number of Pools | 10 |
| Number of Tests | 10 |
| Pools Testing Positive | 9 |
| Tests “Wasted” | 1 |
| Test Round | 2 |
| Number of Pools | 0 |
| Number of Tests | 90* |
| Tests Returning Positive | 9 |
| Tests “Wasted” | 91 |
| Total Number of Tests | 100 |
| Tests Saved | 0 |
*Each of the nine pools is being rerun for individual tests because of an assumption of uniform distribution of viral RNA through the mixture.
It follows then that as pre-test probability of positivity rises, there arrives a time where there is a theoretical mathematical possibility of saving no test kits or time. In fact, an important element of RT-PCR in general is that the saving in time does not scale linearly with the saving in reagents. This is because there are some time-consuming steps like viral RNA extraction, machine setup, maintenance and the like that can be common to all methods, whether pooled- or individual – based. In that regard, in a situation where the pre-test probability of finding positive cases is high, one can actually lose time doing pooling. There are certainly many optimisation algorithms to evade some of these constraints. The Ghana model for instance could be enhanced by successive division of pools instead of complete retesting of every pool that tests positive. But there is only so much optimisation one can do to push back the theoretical limits.
The insight illustrated above has led many experimentalists to argue that when the confirmed cases are above one or two percent of total tested, pooled testing may not be advisable. The only government that has reviewed the situation extensively and taken a policy and ethical (more on “ethicality” later) stance on pooled sampling/testing so far is the Government of India, which has decided to limit the pooling size to 5 samples and the context of use to “screening”, which is most beneficial during the early stages of community transmission of the disease. Which brings us to the last two important caveats.
Error replication
Once the testing process moves out of clinics into the broader community, through for instance the community screening and contact tracing models, the whole affair becomes a complicated supply chain puzzle to unknot.
Public health officers have to be equipped with sample collection devices and sent off to various corners of the country to collect samples. A single officer may collect more than ten samples and there could be more than 1000 sample takers. The samples have to be transported in cold boxes, deposited at aggregation points and finally delivered to labs, which now have to manage these samples as one would any inventory process, with planning for storage, handling, batching and tracking.
Even in the most rigorous of mass testing situations, errors will occur, and far more than would be the case in a routine testing situation. Some samples will be poorly collected and transported than others.
Pooling, in these circumstances, can easily obscure traceability by making it highly difficult to identify points within the supply chain that are defective, since for the vast majority of tests, there is no link between a test result and its supply chain history.
Should a sample be cross-contaminated, the effect is not just on that second sample but on an entire pool. Quite apart from interfering with corrective action protocols, pooled sampling can increase workload due to error replication.
In effect, whilst pooling under laboratory conditions can be heavily controlled to remove error effects, additional safeguards are required in mass testing protocols where samples are not collected inside health facilities.
But by far the most critical issues are ethical in nature.
Ethical clearance
In the pooled sampling and testing methodology, pools that test negative are not retested, for obvious reasons: the whole point is to save time and consumables.
In some cases, this is not an issue, but in others it is. During an epidemic or pandemic, such as the current one, testing is not a monolithic affair. We must look at the mass testing protocol in every country as a multi-layered one involving different cohorts of testing subjects.
First, there are those who were ill or felt ill and reported to a clinic or other health setting. The clinician suspected Covid-19 and sent off samples for testing, a process often referred to as “routine surveillance”. The pre-test probability in such cases (what we might also call the “index of suspicion”) is relatively high. In some cases, despite a negative test result, considering the inherent imperfection of all tests, re-testing is imperative. An example would be a situation where the individual’s symptoms and travel history are so similar to those who typically test positive for the disease. In these circumstances, pooling can be harmful to such patients because negative results are not reviewed individually.
Second, there are those who come into contact with persons who have been confirmed to have the disease. In a country with a sophisticated contact tracing system, the risk level for such persons can be considered elevated if they have been in contact with multiple positively tested individuals in settings that heighten exposure. Here too, despite a negative result, it might be necessary to re-test but in a pooling scenario that would be impractical.
Lastly, we have those individuals who fit some theoretically determined risk profile (for example, living in a neighbourhood where many people have tested positive). Testing in such “community screening” scenarios is driven largely by the evolving picture of the outbreak and by the quality of data, modelling and analysis. In countries where public health institutions are weak, the data, models and analyses involved may well also be weak.
In Ghana’s case, the empirically observed situation accords cleanly with the above descriptions. Using the 21st April 2020 Covid-19 situational report for the Greater Accra Region (the capital city region that is presently the epicenter of the outbreak in Ghana, accounting for more than 85% of all infections), we computed a positive case rate of 8.5% for test subjects selected through routine surveillance and 8% for those selected through contact tracing. Community screening results on the other hand yielded positive cases of less than 0.1% of total number of individuals tested.
Ghana’s fascinatingly low 1.5% rate could thus be the result of a major dilution effect from potentially weak screening. If the community screening results are removed, the Ghanaian observed prevalence rate becomes almost identical with that of neighbours such as Nigeria and Ivory Coast.
Pooled sampling should be used selectively
But even if the community screening exercise is based on perfectly sound models, and I do admit that the current positive rate from routine surveillance nationwide in Ghana is around 2.5% (most likely due to loosening surveillance standards especially outside the capital) and thus less dramatically divergent from the community screening results, individuals screened under this method have different risk profiles and therefore clinical needs.
Those tested under the routine surveillance route, clearly because they are more likely to be sick, and some of those tested through the contact tracing route, because their risk is higher, may all have a right to a standard of care involving re-testing where necessary. Grouping of test subjects into cohorts based on various indices of suspicion and vulnerability seem therefore like an elementary ethical requirement.
In summary, whilst pooled testing could considerably boost testing capacities around the African continent, it should only be implemented in each country following peer-reviewed studies to establish the right optimisation models and after national ethical clearance has established the right of patients to differential diagnosis.
It is probably best then that such methods are reserved for community screening (typically more than 60% of the overall testing volume anyway) only. The evidence shows clearly that this category of testing has the lowest risk, at least before there has been widespread community infection. In the same vein, it may be prudent to avoid using the pooled technique on samples from individuals or patients identified through routine surveillance and contact tracing.
The author, Bright Simons, is a Ghanaian social innovator, entrepreneur, writer and vice-president at IMANI. He is also the founder and president of mPedigree.
Latest Stories
-
Postecoglou backs Bentancur appeal after ‘mistake’
14 mins -
#Manifesto debate: NDC to enact and pass National Climate Law – Prof Klutse
23 mins -
‘Everything a manager could wish for’ – Guardiola signs new deal
33 mins -
TEWU suspends strike after NLC directive, urges swift resolution of grievances
40 mins -
Netflix debuts Grain Media’s explosive film
1 hour -
‘Expired’ rice scandal: FDA is complicit; top officials must be fired – Ablakwa
2 hours -
#TheManifestoDebate: We’ll provide potable water, expand water distribution network – NDC
2 hours -
IPR Ghana@50: Pupils educated to keep the environment clean
2 hours -
PenTrust CEO named ‘Best Pensions CEO’, company wins ‘Scheme Administrator Award’ at Ghana Accountancy & Finance Awards 2024
2 hours -
Alan Kyerematen’s ‘Brighter Future for Health Professionals’ in Ghana Revealed in Bono
3 hours -
#TheManifestoDebate: NPP will ensure a safer, cleaner and greener environment – Dr Kokofu
3 hours -
2024 Election: Police to deal with individuals who will cause trouble – IGP
3 hours -
Seychelles President’s visit rekindles historical and diplomatic ties with Ghana
3 hours -
Election 2024: EC destroys defective ballot papers for Ahafo and Volta regions
3 hours -
2024 Election: I am sad EC disqualified me, but I endorse CPP’s candidate – PNP’s Nabla
3 hours

